
This means that line spectra can be used to identify elements.Ĭontinuous spectra are produced by electrons being shared between many atoms, giving a huge range of possible frequencies, as shown below. As a result each produces photons with different energy and so the line spectra for different elements will be different. This is not a continuous spectrum as only light of specific frequencies and specific colours are produced.ĭifferent types of atoms have different energy levels. It assumes that you know how these spectra arise, and know what is meant by terms such as absorbance, molar absorptivity and lambda-max. This causes line emission spectra to be produced, as shown below. This page takes a brief look at how UV-visible absorption spectra can be used to help identify compounds and to measure the concentrations of colored solutions. Bottom: PAR action spectrum (oxygen evolution per incident photon) of an isolated chloroplast.
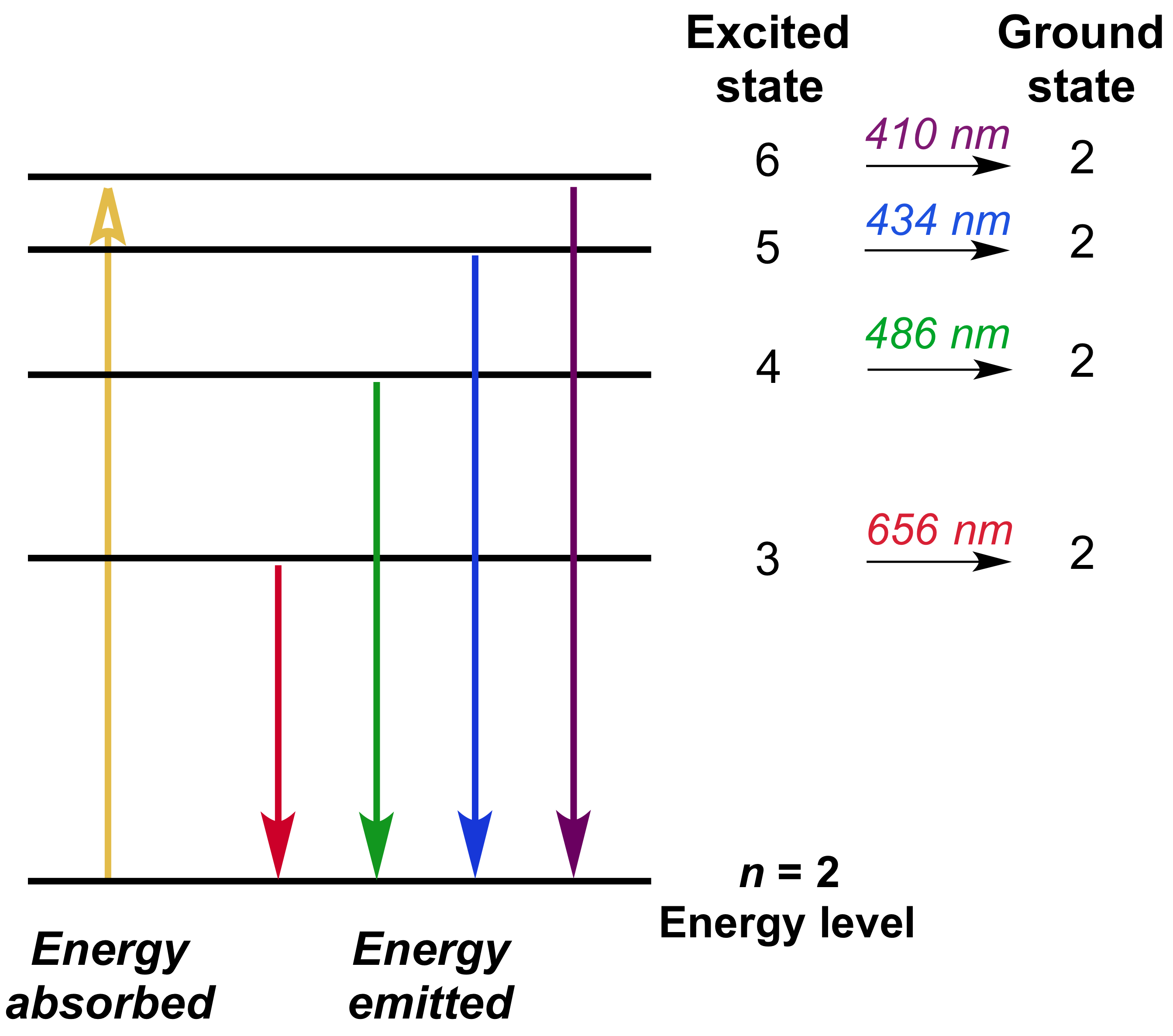
This means that each electron transition will produce a photon of a different frequency and hence a different colour. Top: Absorption spectra for chlorophyll-A, chlorophyll-B, and carotenoids extracted in a solution.

If an electron is in an excited state it can return to a lower energy level. Absorption spectrum graphs show us the wavelengths of light that different photosynthetic pigments absorb.


 0 kommentar(er)
0 kommentar(er)
